Kerns, D.L., M.E. Matheron, J.C. Palumbo, C.A. Sanchez, D.W. Still, B.R. Tickes, K. Umeda and M.A. Wilcox. 2/99. Guidelines for Head Lettuce Production in Arizona. IPM Series Number 12. Publication number az1099. Cooperative Extension, College of Agriculture and Life Sciences, University of Arizona, Tucson, Arizona. URL: http://cals.arizona.edu/crops/vegetables/cropmgt/az1099.html
Introduction
Lettuce, (Lactuca sativa L.), is native to the Mediterranean area and was domesticated in Egypt around 4,500 B.C. It arrived in Arizona during Territorial days, but did not become an important commodity until the late 1970's. Today, Arizona ranks second, following California, in production of lettuce. Lettuce production in Arizona includes head, leaf and romaine lettuces, and is the State's leading cash crop averaging more than $300 million in value. There are approximately 75,000 acres of lettuce grown in the southwestern United States.
Production Areas
Approximately 45,000-55,000 acres of the lettuce are grown in Arizona, 95% of which is produced in the lower Colorado River and Gila River Valleys of Yuma County where elevation is below 100 feet (Fig. 1).
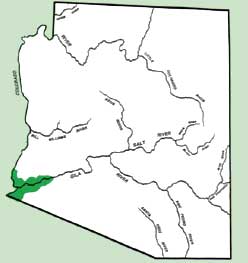
Figure 1. Primary area of lettuce production in Arizona.
Other production areas include Cochise County in southeastern Arizona, and Maricopa, Pinal and Pima Counties in central Arizona.
Yuma County is characterized by having hot summer temperatures and moderate winter temperatures. Summer temperatures average 87° F with an average daily maximum of 104° F. In winter, the average temperature is 55° F and average daily minimum is 40° F. Rainfall in Yuma County averages less than 3 inches annually, most of which falls during August and September.
Soil and Seedbed Preparation
Lettuce in Arizona is produced on a range of soil types. The lighter textured soils may include loamy sands and sandy loams while the heavier soils would include clay loams. Much of the land used to produce lettuce or other cool season vegetables is used for other warm season crops during the spring, summer, or fall period. Crops typically grown between produce crops may include wheat, melons, safflower, cotton, or sudangrass.
Lettuce grows best in fields that are level and well drained. For this reason, land cropped to lettuce is typically deep chiseled and laser leveled every season. Deep chiseling helps facilitate good internal drainage on even the heaviest of soils and helps preclude detrimental salt accumulation in the soil profile. Lettuce is highly sensitive to salinity. Soils with an electrical conductivity of their saturated paste extract of two or less are highly desirable. Land used for lettuce is often pre-irrigated before land preparation is completed to facilitate salt leaching (movement with water below the root zone).
The seedbed used for lettuce should be tilled until it is soft and friable to a depth of 10 to 12 inches. Depending on soil texture, this is accomplished by moderate to extensive disking. Once the soil is of a desirable consistency, rows are listed, beds are shaped, and lettuce is seeded two rows per bed. In Arizona, lettuce beds are typically 8 to 10 inches high on 40 to 42 inch centers and are almost always laid out in a north-south direction. This orientation minimizes temperature and light differences between the two rows on each bed.
Variety and Seed Selection and Seed Handling
A great number of varieties are successfully grown in the lower desert of Arizona. New varieties are released or removed from commercial production each year, making it impractical to list them in this publication. The variety a grower may use is based both upon physiological considerations and personal preferences for a particular variety. All varieties are bred for early vigor, size, earliness and uniformity of maturity, shape, texture, pest resistance and so forth. For more specific information regarding varieties in your area, consult your local county Cooperative Extension Office or a seed dealer. For planting purposes, the lettuce season is typically divided into three categories, early (late-August-September 30; known as the 'Empire' slot), mid (October 1-25; known as the 'Winterhaven' slot), and late (October 25-December 10; known as the 'Van Guard' slot).
The primary physiological considerations for selecting a variety are seed germination temperature, length of growing season, and day length sensitivity. Depending on variety, germination is inhibited by temperatures of 77 to 95° F. The prevention of germination due to high temperatures is known as thermoinhibition. A thermoinhibited lettuce seed will germinate when returned to more suitable temperatures. However, continued exposure to high temperatures may induce a secondary dormancy, called thermodormancy. A thermodormant seed will not germinate even when returned to non-inhibiting temperatures. Germination of lettuce may be promoted by light and inhibited by dark; this is known as photodormancy. Specifically, far-red light inhibits germination while red light (abundant sunlight) promotes germination. The leaf-lettuce varieties are often photodormant.
Both thermodormancy and photodormancy may be alleviated by priming. Priming, also know as osmopriming or osmoconditioning, is a controlled hydration process in which the amount of water seeds imbibe is controlled by an osmoticum, typically polyethylene glycol. During priming the seed imbibes enough water to start many of the physiological processes associated with germination, but not enough to cause the radicle to protrude, or the seed to germinate. In addition to alleviating thermo- and photo-dormancy, priming shortens the germination time and synchronizes germination, thus leading to more uniform stands.
Although only certain varieties are primed, virtually all lettuce seed is pelletized (Fig. 2). During this process an inert material, usually diatomaceous earth, is coated around individual lettuce seeds. This allows singularization of the seed during planting. Although necessary, both the priming and pelleting process lead to shortened shelf life, compared to a seed that has not been similarly treated. The cause of this is not currently known, but new technologies are being developed which minimize these negative effects.

Figure 2. Pelletized (right) and raw lettuce seed (left).
Seed deterioration is a naturally occurring phenomenon, but steps may be taken to minimize this process. Seed should be kept at cool temperatures and not exposed to high relative humidity. A good rule of thumb is the 50/50 rule, which states that the combination of temperature (°F) and humidity should not exceed 100. Seed exposed to temperatures above 104° F for as little as two days will have demonstrably lower vigor. The amount of time seed is allowed to remain in the back of pickups, on loading docks, or on a dry seed bed, should be minimized, as it will contribute to accelerated seed deterioration resulting in lowered vigor, and eventually low overall germination.
Planting Procedures
In Arizona, most lettuce is seeded using a StanHay type planter (Fig. 3). These are precision planters that utilize rubber belts with small holes spaced to deliver a single pelletized seed. Holes in the rubber belts are usually spaced 1-1 « inches apart. However, some growers prefer to plant to stand to reduce thinning costs. Seed is usually planted at a depth of 1/8 to 3/8 inches with 10 to 15% of the seed being visible in the seed row after planting. Pelletized seed is planted at the rate of 6 to 8 pounds per acre which should result in about 6 to 12 seeds per foot of row. This will result in a plant population of 20,000 to 26,000 plants per acre after thinning. The seeding rate is largely a function of the planting date, method of irrigation and variety to be planted. The crop should be thinned, after the first two true leaves have developed, to approximately 10 to 14 inches between plants depending upon the head size of the variety planted.
Planting date is primarily a function of the variety and desired harvest date. Planting begins as early as late August and continues into the middle of December. Plantings prior to October 15 are considered to be fall lettuce, and later plantings are considered spring lettuce. Harvesting takes place primarily from December through March. Optimum germination and growing temperatures vary depending upon the variety planted.

Figure 3. Lettuce planter.
Growth and Development
Lettuce passes through six distinct development stages: seed, cotyledon, seedling, rosette, cupping and heading periods. The seed stage occurs from pre-planting to emergence. Once exposed to water and appropriate temperature, the seed will begin germination, usually requiring as little as 12 hours for fall planted varieties or up to 7 days for winter planted varieties. Once the plant sheds the seed coat and emerges from the soil, it enters the cotyledon stage. The cotyledon stage last until the plant is able to better establish its roots. Once the root has grown a couple of inches, the seedling begins to grow upwards and the first true leaf emerges. From emergence from the soil to the first true leaf usually requires approximately 7 days for fall planted lettuce and 20 days for winter planted lettuce. The seedling stage occurs from the first true leaf until the plant develops a distinct circular cluster of leaves known as a rosette. The rosette stage for fall planted lettuce will generally last 25 days, but may last as long as 50 days for winter planted lettuce. Cupping begins when the tips of the inner leaves begin to curl inwards on the edges. Cupping signifies that the beginning of head formation is near, and will usually last about 7 days for fall planted lettuce and 14 days for winter planted varieties. Heading begins once the cupped leaves begin to overlap and cover the growing point of the plant. Head formation will continue until the crop is ready for harvest, which generally last about 30 days for fall planted varieties and about 45 days for winter planted varieties. Fall planted lettuce may require as little as 65 days from the beginning of germination to harvest, while winter planted lettuce will require as long as 120 days.
Fertilization
Lettuce produced on low desert soils requires from 150 to 300 pounds of nitrogen (N) to the acre for optimal yields. The actual rate will vary depending upon residual soil N, soil texture, irrigation and rainfall. Split applications of N are usually more efficient than a single preplant application because N in the soil is subject to leaching, denitrification (gaseous loss to the atmosphere), and other mechanisms of loss during the growing season. Generally, 50 pounds N per acre is considered adequate preplant N. Stand loss and stunting may result from excessive amounts of ammonium-N (especially on light or sandy soils).
Subsequent applications of N can be applied by sidedress or water-run and usually start after thinning and cultivation. Typically following thinning, lettuce will be cultivated and sidedressed with N. The amount and frequency of N application can be adjusted based upon a pre-sidedress soil nitrate-N test or a plant midrib tissue test. Lettuce grown in soils with a nitrate-N concentration of 20 ppm or greater in the top 12 inches will generally not respond to additional N fertilizer. Midrib samples are also useful during the second half of the growing season (after the 8 to 10-leaf stage). Generally, lettuce is not considered deficient in N if midribs nitrate-N concentrations exceed 8000 ppm. A second series of cultivation and sidedressing is usually conducted approximately 14 days following the first such operation, usually near early head formation. Additional sidedress applications of N may be applied if adverse growing conditions are encountered.
Recent research indicates that the use of controlled release fertilizers may represent a viable alternative to split application of conventional soluble N sources. There is considerable variation in N release rates and costs of controlled release N fertilizers, and individual products should be closely evaluated before they are utilized.
Phosphorus (P) fertilizer should be broadcast or banded 2 to 3 inches below and beside the seed row immediately before planting. Studies have shown that P applied later in the season is less effective than that applied preplant. Rates of P applied can be adjusted using a preplant P soil test. Lettuce in Arizona will require from 200 to 400 lbs P2O5 per acre. Because P applied by band is utilized more efficiently, generally only 50 to 60% of that typically applied by broadcasting is needed for optimal yields. Tissue tests are considered inconsequential because it is extremely difficult to correct P deficiencies mid-season. Nevertheless, these tests are useful tools to diagnose P deficiencies. Generally, whole plants with leaf tissue P concentrations of less than 0.38% or midrib P extracts less than 0.30% are considered deficient in P.
Potassium fertilization is generally not needed for lettuce production in Arizona. Most soils used for lettuce production in Arizona have exchangeable K levels, a clay mineralogy (mica), and K release rates that make a K response improbable. Additionally, most lettuce is irrigated with Colorado River water that contains approximately 5 ppm K or 15 lbs K per acre foot of water.
Although some growers commonly use micronutrient fertilizers, most recent research suggests that lettuce produced in Arizona does not show a positive yield and quality response. Therefore, the routine use of soil or foliar applied micronutrients cannot be economically justified. Micronutrient fertilization should be based on the actual diagnosis of a deficiency.
Cooperative Extension bulletin number 8922 "Fertilizing Head Lettuce in Arizona" provides tables for recommended fertilizer rates (based upon soil and midrib analysis) and detailed sampling procedures. Contact your local county Cooperative Extension office for a copy of this publication.
Irrigation
Generally, 38 to 50 inches of water per acre are required to produce a desirable lettuce crop, but this varies dramatically with soil type, slope of field, temperatures and planting window. Irrigation water is delivered via canals from the Colorado River, or from deep wells, in Yuma County.
There are two distinct irrigation stages in the low desert of Arizona. The first irrigation stage involves seed germination and stand establishment. During the 'Empire' slot in late August and early September, seed bed temperatures may exceed 140 øF. Under these conditions, impact sprinklers are essential to not only provide water, but also to reduce the temperature of the seed bed though evaporative cooling (Fig. 4).

Figure 4. Sprinklers are commonly used to germinate lettuce
seed when temperatures are hot.
When used, sprinkler pipe is placed in the field immediately after planting. The pipes are typically 3 inches in diameter and are spaced 40 ft apart. Each pipe contains 12 inch risers spaced 30 ft apart, mounted with a 4 inch long impact sprinkler head. In Yuma, Weather Tec 10-10 or Rainbird 14-VH sprinkler heads are most commonly used. The sprinkler heads are typically equipped with a 5/64 inch orifice to disperse the water. Water pressure is maintained at 60 to 80 psi, depending on the temperature and evaporation rate. Worn orifices or inadequate water pressure may result in excessively large water droplet sizes that may wash out seed planted in the immediate vicinity of the sprinkler head. During periods of high temperatures, growers may opt to use impact sprinkler heads with a 23° v-shaped wedge drive water diffuser. The wedge drive reduces droplet size, increases evaporative cooling and speeds the sprinkler's rotation. Under hot conditions, sprinklers are continuously kept running unless the field is in jeopardy of becoming super saturated or anaerobic, in which case every other sprinkler pipe may be turned off and/or all turned off during the night and morning.
Under cooler conditions, evaporative cooling may not be an important consideration, but sprinklers are often used to deliver uniform moisture to maximize stand uniformity. Under these conditions, sprinklers are turned on as needed to maintain a moist seed bed. When using sprinklers, the water source must be low (800 parts per million {ppm} or less) in sodium and chloride salts. After stand establishment, the field is allowed to dry and the sprinkler pipes removed.
In addition to using sprinklers for stand establishment, once daily temperatures cool to less than 85° F, lettuce may be germinated using furrow irrigation. For effective germination, beds should be moistened thoroughly but not to the point that they become super saturated and anaerobic. The field is generally maintained in a flooded condition until the lettuce has uniformly emerged.
The second irrigation stage involves furrow irrigation for crop maintenance following stand establishment. Usually an irrigation is made to soften the soil prior to thinning and cultivation. Afterwards, adequate moisture should be maintained throughout plant development.
During the second irrigation stage, the furrows are typically filled within approximately 1 to 2 inches from the top of the beds and then the water is cut off. Care is taken to avoid saturating the middle of the bed to the point where the top of the bed becomes wet or "blackened." Otherwise, the potential of developing infections of bottom rot, Rhizoctonia solani, or leaf drop, Sclerotinia spp., will be greatly enhanced. During harvest, an irrigation may be necessary between cuttings if multiple harvests are necessary or profitable.
Cultivation
Typically following thinning, lettuce will be cultivated for weeds, worked with a rotary spiker to remove some weeds, breakup the soil crust, sidedressed with N, and then furrowed out or "bolused." The spiker removes some weeds, but is primarily used to breakup the crust and restore friability to the soil before sidedressing. The bolus is used to restore the shape of the beds and allow for more uniform irrigation. The bolus implement consists of two chisels situated about 3 inches of the center of each furrow to breakup the soil pan. The chisels are followed by a wedge to move displaced soil back onto the sides of the furrows, then followed by a large steel wheel to pack and shape the furrow. Approximately 14 days following the first cultivation, near early head formation, a second series of spiking, sidedressing and bolusing is often performed.
Weed Control
Economic losses due to weeds can be a serious problem in the production of lettuce. With planting taking place from late August through December, summer annual, winter annual and perennial weeds may be a problem. Weeds decrease crop yield and quality through competition for water, nutrients and sunlight. In addition, many weeds harbor destructive insect pests and serve as alternative hosts for other organisms which cause crop diseases.
Weeds commonly encountered in lettuce in Yuma, AZ in the late summer plantings include summer annual grasses such as watergrass or junglerice (Echinochloa crus-galli or E. colona) and sprangletop (Leptochola spp.); and small-seeded broadleaved weeds including purslane (Portulaca oleracea), pigweeds (Amaranthus spp.) And groundcherry (Physalis wrightii) In the transition period between hot and cooler temperatures, lambsquarters (Chenopodium spp.), nettleleaf goosefoot (Chenopodium murale), knotweed (Polygonum spp.) And cheeseweed (Malva spp.) become difficult to control. By late-October when temperatures cool, winter annual weeds are prevalent. Winter annual grasses include canarygrass (Phalaris spp.), wild oat (Avena fatua), annual bluegrass (Poa annua), wild barleys (Hordeum spp.) And volunteer small grains. Cruciferous weeds such as London rocket (Sisymbrium irio), shepherdspurse (Capsella bursapastoris) and black mustard (Brassica nigra) are commonly encountered. Lettuce-related weed species are also prevalent under cooler conditions, including prickly lettuce (Lactuca serriola) and sowthistle (Sonchus oleraceous).
Cultural and chemical technologies are utilized for weed control in lettuce. Cultural practices including mechanical cultivation and hand hoeing are utilized from the fallow period prior to seed bed formation until first layby.
Herbicides have been used for weed management in lettuce for more than 30 years. There are four distinct timings of application for herbicides in lettuce: fallow, preplant, preemergence and postemergence.
Fallow weeds
Before seedbed preparation, Roundup Ultra (glyphosate), applied at 0.375 to 5.0 lbs-a.i. (active ingredient) per acre and Gramoxone Extra (paraquat) applied at 0.5 to 1.0 lbs-a.i. per acre, are often used to reduce weed seed banks. Fields are laser leveled and then flushed with water to germinate weed seeds. These broadspectrum non-selective herbicides are applied after sufficient weeds have emerged.
Preplant
After a field has been laser leveled and prior to listing the seedbeds, Balan (benefin), at 1.2 to 1.5 lbs-a.i. Per acre, is commonly applied and disked into the top 4 to 6 inches of the soil. Balan controls some grasses and some small-seeded broadleaved weeds (Table 1). Balan moves very little in the soil profile or within the plant tissue.
| Balan | Kerb | Prefar | |
|---|---|---|---|
| Excellent | Partial | Excellent | Summer Grasses |
| Excellent | Partial | Excellent | Pigweed |
| Excellent | Excellent | Excellent | Purslane |
| Partial | Poor | Partial | Groundcherry |
| Excellent | Excellent | Poor | Goosefoot |
| Excellent | Excellent | Poor | Lambsquarters |
| Partial | Excellent | Partial | London Rocket |
| Partial | Excellent | Partial | Shepardspurse |
| Partial | Partial | Partial | Sowthistle |
| Excellent | Excellent | Poor | Knotweed |
| Poor | Excellent | Partial | Cereals |
| Poor | Excellent | Partial | Wildoat |
| Excellent | Excellent | Excellent | Canarygrass |
| Excellent | Excellent | Excellent | Bluegrass |
Table 1. Efficacy of preplant and preemergence herbicides
against commonly occurring weeds in lettuce.
Preplant/Preemergence
Kerb (pronamide), at 1.0 to 2.0 lbs-a.i. Per acre, can be used preplant, preemergence or postemergence. However, most applications are applied banded over the top of the seedbed just before or immediately following planting. These preplant and preemergence applications should immediately be followed by sprinkler irrigation of 1 to 2 inches. However, Kerb is sensitive to movement in the soil with water, and excessive sprinkler irrigation will leach the material out of the weed seed zone (top 1 inch of soil) and render the product ineffective. Thus, the effectiveness of Kerb is greatest if used on lettuce that is not sprinkle irrigated. For postemergence applications, apply before or after lettuce emerges, but prior to weed emergence. Kerb is most effective against cool season annuals, and best fits lettuce planted after September (Table 1).
Prefar (bensulide); at 5.0 to 6.0 lbs-ai per acre, is often used in preplant or preemergence for control of selected grasses and broadleaved weeds (Table 1). Prefar is most effective against summer annual weeds, and is most efficient in lettuce planted in August and September. Preplant applications should be mechanically incorporated 1 to 2 inches before planting. Preemergence applications should be immediately followed by irrigation. When incorporating using sprinkler irrigation, wet the soil to the depth of 2 to 4 inches. Furrow irrigation should thoroughly wet, or "blackened," the top of the bed. Prefar moves very little in the soil and should stay within the weed seed zone. Prefar can also be applied through sprinkler irrigation.
Maximum weed control using preplant or preemergence herbicide is best achieved if the herbicide can be concentrated within the weed seed germination zone (top 1 to 2 inches of soil). Additionally, a smooth, clod-free surface will maximize weed control when using Kerb or Prefar. Combinations of Balan and Kerb control most weeds. Use Balan preplant incorporated followed by Kerb applied preemergence and incorporated by irrigation , or apply both herbicides preplant and mechanically incorporate. Weeds related to lettuce such as prickly lettuce and sowthistle are not controlled. Other difficult to control weeds that may occur in lettuce include horseweed (Conyza canadensis), fleabane (Conyza bonariensis), cudweed (Gnaphalium palustre), groundsel (Senecio spp.), annual clovers (Leguminosae), filaree (Erodium spp.) And broadleaf perennials and nutsedges (Cyperus spp.).
Balan, Kerb and Prefar can cause injury to lettuce, especially under environmental conditions where lettuce emergence is slowed. Also, some lettuce varieties are more prone to injury than others. Refer to the herbicide label for details. For more information concerning herbicide damage to lettuce, refer to the University of Arizona Cooperative Extension IPM Series Publication number 9, entitled "Lettuce Injury from Preplant and Preemergence Herbicides."
Postemergence
Poast (sethoxydim) used at 0.1 to 0.3 lbs-a.i. Per acre with a crop oil concentrate added will selectively control most annual and perennial grasses found infesting lettuce. Timing is critical for optimal control; apply Poast when grasses are small. Do not apply to grasses under stress. Thorough coverage is required. Do not tank mix with other pesticides as efficacy may be reduced. Do not cultivate within 5 days prior to application or within 7 days following application. See the label for specific directions.
Insect/Pest Management
There are many insecticides registered for use in lettuce,
more than can be adequately addressed in this document. Refer to specific
insecticide labels for details. For more comprehensive information concerning
the description, development, and management of insects infesting lettuce
and other vegetable crops, refer to the following University of Arizona
Cooperative Extension Publications: number 195007, "Insect Pest Management
Guidelines for Cole Crops, Cucurbits, Lettuce, and Leafy Green Vegetables,"
number 195017, "Using Admire on Desert
Vegetable Crops," and number
197008, Confirm and Success: New Tools for Insect Management in Cole Crops
and Leafy Green Vegetables in Arizona."
| Crickets & Beetles Saltmarsh Caterpillar Sweetpotato Whitefly Leafminers Beet Armyworm |
Cabbage Looper Corn Earworm Tobacco Budworm Green Peach Aphid Thrips |
Crickets and Ground Dwelling Beetles
Field crickets (Gryllus spp.), Darkling Beetles (Blapstinus spp.), Ground Beetles (Carabids) and Rove Beetles (Staphylinids)
Description and Life History: These insects are annual pests in early planted sprinkler-irrigated lettuce fields in the low desert. When they occur, they can quickly destroy most of a field. Problems are usually in fields planted closely to cotton or Sudangrass in August and September. Moving out of cotton, Sudangrass and desert flora, large numbers of these pests will migrate to seedling lettuce if available. Most damage occurs at night. They hide during the day in soil cracks, ditches, weeds, and under irrigation pipes.
Darkling beetles are shiny dark black or brown and approximately 1/4 inch in length. They are similar in appearance to many ground beetles. Darkling beetles normally have the tips of their antennae slightly enlarged, while ground beetles antennae are not enlarged on the tips. Most ground beetles encountered in lettuce are about ¬ inch in length and are black, brown, or reddish in color. Ground beetles are predators feeding primarily on other insects.
Rove beetles are mostly small elongated beetles less than ¬ inch in length and shiny dark black or brown. They have very short elytra covering their wings, but their abdomen is not covered. Rove beetles are often confused with winged ants or termites. When disturbed they will elevate their abdomens similarly to a scorpion. Rove beetles are insect predators or scavengers feeding on debris in the field.
Damage: Cricket and darkling beetles will destroy a crop by eating the newly emerged seedlings. Although ground beetles and rove beetles do not feed on the plants and are usually considered beneficial insects, they often damage fall vegetable crops by digging and rooting up the seed and small seedlings.
Management and Control: These insects are difficult to monitor. Early-planted lettuce in close proximity to cotton or Sudangrass should be considered high risk fields and should probably be treated as soon as the seed begins germinating.
Apply baits around field edges to control migrating populations and apply insecticides through the sprinkler pipe during germination and when the plants emerge. Scout the field by looking under the sprinkler pipe to determine if control was achieved or if re-application is necessary.
Saltmarsh Caterpillar, Estigmene acrea (Drury)
Description and Life History: Saltmarsh caterpillars are not normally a pest of fall grown vegetables but will often migrate as larvae from neighboring cotton or alfalfa. Large populations can be extremely damaging to seedling lettuce.
The larvae are usually yellowish brown in color and covered with long, dark black and red hair. Many people refer to them as wooly bear caterpillars. Full-grown larvae may be 2 inches long. Adult moths have white to yellowish wings and are peppered with many black spots. Their wing span is approximately 2 inches Eggs are laid in clusters of 20 or more on the leaves
Damage: Most damage occurs to early planted seedling lettuce. Large populations of larvae will move out of newly defoliated cotton and devour the young plants. After thinning, saltmarsh caterpillars are generally not a problem. However, they should be included in counts for Lepidopterous larvae. On older plants damage is distinctive. They prefer to feed in groups and will completely skeletonize several adjacent plants.
Management and Control: Scout adjacent cotton fields prior to lettuce emergence. It is best to control saltmarsh caterpillars before they enter the field. If possible treat the population in the cotton field during or just before defoliation. Saltmarsh caterpillars are particularly sensitive to Bacillus thuringiensis (B.t.).
Physical barriers are effective at preventing larvae from entering a field. Saltmarsh caterpillars do not like to cross fence type barriers of aluminum sheeting or irrigation pipe. These devises can be used to herd populations into holes containing cups of oil. Ditches filled with water containing liquid detergent or oil are also effective. Carbaryl can be sprayed around cotton fields or along ditches to kill migrating populations.
Sweetpotato Whitefly, Bemisia tabaci (Gennadius) B-Strain a.k.a Silverleaf Whitefly, Bemisia agentifolii Bellows and Perring
Description and Life Cycle: In the Southwest, the sweetpotato whitefly is one of the principal pests of crops (Fig. 5). It was not considered an important pest until the early 1980's, when extremely large populations became common on cotton, melons and lettuce throughout the Southwest. In a short period of time, the sweetpotato whitefly shifted from a position as a secondary pest to being the primary pest for fall vegetables. This shift in pest status is thought to have occurred due to the introduction of a new strain of sweetpotato whitefly (B-strain). The B-strain is also known as the silverleaf whitefly. The host range of the B-strain is much greater than the old strain.

Figure 5. Sweetpotato whitefly adults, nymphs and eggs.
The eggs are deposited mainly on the underside of leaves. The eggs are minute, pointed, oblong, and yellow. One female will lay numerous eggs. Near hatching, the apex of the egg will darken. Eggs hatch in 2 to 5 days into crawlers with limited mobility. Crawlers (first instar nymphs) are yellowish in color and are oval and flattened in appearance. They are less than 1/10 inch in length, and will move about until they locate a minor vein. Once they locate an acceptable feeding site, they become immobile and remain so through four nymphal instars. They feed by inserting their tubular mouthparts into the vein and extracting phloem sap. Late third and fourth instar nymphs have distinctive red eye spots and are termed red-eyed nymphs. At the end of the fourth instar they enter what is called the pupal stage. Their pupal cases are dome shaped and oval in their outline, and are quite small. Following the pupal stage, fairly mobile adults emerge. These are capable of easily moving as far as 1 « mile from where they originated. As a consequence the size of the whitefly population in a field of fall lettuce is related, in a large part, to the proximity of the crop to cotton or melon fields.
Damage: Damage by large whitefly populations can result in crop injury by reducing head size, delaying harvest, and leaf chlorosis associated with whitefly feeding. Whiteflies also cause economic damage through contamination associated with the insect themselves, honeydew and sooty mold accumulation. Total destruction of early fall lettuce plantings has been observed because whiteflies have extracted large amounts of phloem sap from seedlings.
Management and Control: Lettuce planted in high risk situations (August and September plantings, or later plantings near a significant whitefly source) should be treated prophylactically with a soil-applied systemic insecticide. Lettuce planted in October, or later when temperatures have declined, should be treated as needed with foliar adulticides. Best control is usually achieved from tank mixing insecticides. Good coverage is essential for adequate control.
Lettuce should be monitored as soon as the plants emerge. Whitefly populations will build in cotton and alfalfa, so growers should pay particularly close attention to lettuce planted downwind or adjacent to these fields. Once whitefly adults appear in a field in sufficient numbers, treatments should begin. Whiteflies are best controlled by preventing colonization; do not allow adults to build and lay eggs. Monitor for whiteflies early in the morning when the adults are sedentary. Once temperatures begin to increase, the adults will begin to stir and move, and they will become difficult to count. During mid-morning, monitor movement by looking for dispersing swarms.
Delaying plantings of fall lettuce until after most cotton has been defoliated and harvested will avoid major whitefly population flights. Destruction of crops post-harvest is a valuable practice for preventing whitefly population escalation. Once temperatures begin to cool, whiteflies are generally not a problem on lettuce. Thus, spring lettuce is generally not affected by whiteflies.
Leafminers
Vegetable Leafminer, Liriomyza sativae Blanchard and Liriomyza trifolii (Burgess)
Description and Life History: Liriomyza leafminers occasionally cause economic damage to lettuce. The principal leafminer species in Arizona include L. trifolii and the vegetable leafminer, L. sativae (Fig. 6). Problems with leafminers are most often prevalent in lettuce planted near cotton or melon fields. On lettuce planted in August or September, L. sativae is usually the predominant species, but by February, L. trifolii usually predominates.

Figure 6. Adult vegetable leafminer fly.
The leafminer adults are small, shiny black and yellow flies with a bright yellow triangular spot on the upper thorax between the wings. Subtle differences in color exist between adult L. sativae and L. trifolii. The latter species has developed resistance to many insecticides. Females puncture young leaves and oviposit eggs within the leaf. Both male and female flies often feed at puncture sites. After 2 to 4 days, larvae hatch and begin feeding on plant mesophyll tissue just below the upper surface of the leaf. The winding tunnels that result are initially small and narrow, but increase in size as the larvae grow. After completing three instars in 4 to 20 days, larvae emerge from the mines and drop to the soil to pupate. Pupation takes 7 to 25 days. At temperatures of 50° F or lower, development ceases.
On lettuce, leafminers sometimes complete the pupal stage on the plant near the base of the leaf.
Adult flies emerge from the pupae after about 7 to 11 days. The entire life cycle can be completed in less than 3 weeks when the temperatures are warm. Many generations are produced each year in Arizona.
Damage: Mining of leaves by the larvae is the principal cause of plant injury. The mines reduce plant photosynthesis, render harvestable portions unmarketable, and provide an access for secondary pathogens. When populations are high, plants may be killed or stressed to the point where pathogens can easily infect the plant. Leafminers can also cause damage after harvest. Leafminers that cut out of the leaf tissue after harvest will sometimes pupate in-between the leaves. These pupae not only act as contaminates, but will often die and rot, providing a substrate for post-harvest pathogens to infect the lettuce.
Management and Control: Monitor young seedlings regularly for the presence of leafminers. In lettuce, most mines occur on the cotyledons and first true leaves. After thinning, sample leaves from the middle portion of the plant. If leafminer populations build to high levels when seedlings have only four or five leaves, chemical treatment may be necessary. The threshold for leafminers in lettuce is an average of one or more active mines per leaf except on the marketable portions where damage cannot be tolerated.
Sticky traps can assist in determining when early migrations take place, and also help in determining species composition. It is important to identify the predominant leafminer species, L. trifoliiis much harder to control with insecticides than L. sativae.
Natural enemies, primarily parasitic wasps in the Diglyphus, Opius and Chrysocharis genera, usually maintain leafminer population below economic injury levels. Parasitoids are often killed by insecticides applied to control other pests such as beet armyworm. This results in a secondary outbreak of leafminers. Use of selective insecticides for control of worms will often preserve leafminer parasitoids so that treatment for leafminers will not be necessary.
Beet Armyworm, Spodoptera exigua (Hubner)
Description and Life History: Beet armyworm is a key pest of lettuce (Fig. 7). In Arizona, it is usually most prevalent from August through November on fall-planted lettuce. However, when temperatures are warm, this pest can be a problem season-long, particularly if alfalfa is nearby. The larvae feed on many field crops, including cotton and alfalfa, and often migrate from these crops onto lettuce in the fall. Several summer annual weeds also serve as hosts.
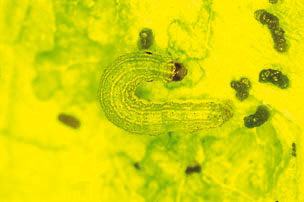
Figure 7. Beet armyworm larva.
Eggs are light green in color and are laid in irregular clumps or masses, usually on the under surface of leaves. One female will lay on average 500 to 600 eggs over a 4 to 10 day period. The female moth covers the eggs with white scales from her body, giving the egg masses a cottony appearance. Eggs will darken as they near hatching, and will hatch in 2 to 5 days. The young larvae will feed in groups and spin webs over the underside of the foliage where they are feeding. Larvae vary in color, but are usually olive green with light-colored stripes down the back and a broader stripe along each side. Beet armyworms usually have a dark spot on the side of the body above the second true leg. Mature larvae vary in size but are usually about 1¬ inches in length. Larvae will generally pass through five instars. The armyworm larvae disperse as they get older and move toward the center of the plant. Large larvae are quite mobile, and a single larva may attack several plants. Larvae reach maturity in about 2 to 3 weeks in warm weather and pupate in the soil. The moth has grayish-brown forewings with a pale spot in the mid-front margin, and the hindwings are white with a dark anterior margin. The wingspan of an adult is approximately 1 1/4 inches The entire life cycle from egg to adult requires approximately 36 days at 80° F.
Damage: Hatching larvae begin feeding on the leaf and may completely consume seedlings. Beet armyworms may severely stunt or kill seedling lettuce plants. Damage to lettuce is usually not economically damaging between thinning and cupping stages unless populations are high. However, once cupping begins, larvae may feed on the head, rendering it unmarketable. Armyworm larvae enter heads from the bottom working their way inward while feeding along the leaf margins. Often the damage cannot be seen without removing frame leaves and dissecting the head.
Management and Control: Cultural controls can help suppress armyworm populations. Disk fields immediately following harvest to kill larvae and pupae. Sanitation along field borders is important; armyworms often migrate from weedy field edges into newly planted fields.
Monitoring for beet armyworm on lettuce should begin before seedlings emerge. Control of beet armyworms on seedling lettuce is essential for stand establishment. Check weeds on ditch banks and field borders for larvae and egg masses as fields are being seeded. Once seedlings emerge, sample at least 25 plants in each quadrant of the field twice a week for armyworm egg masses and young larvae. The action threshold for all Lepidopterous larvae in fall lettuce between thinning and heading is one first or second instar larva for every 50 plants. Many growers have reported difficulty controlling beet armyworms with insecticides, and resistance to Lannate (methomyl) has been documented in Yuma County. Good spray coverage and insecticide resistance management tactics should be practiced. Target small larvae which are easier to control with insecticides. Timing insecticide applications at peak egg hatch will improve control. Addition of a B.t. to conventional insecticides will usually increase control and aid in resistance management. Just before and after heading, treat if Lepidopterous larvae reach one worm per 25 plants.
Cabbage Looper, Trichoplusia ni (Hubner)
Description and Life History: The cabbage looper is a very destructive pest on lettuce and will feed on many other crops including cole crops, leafy greens, melons, tomatoes, and cotton (Fig. 8). Cabbage loopers occur year round in Arizona's central and southwestern desert areas. Populations are especially a problem in the fall, when newly-planted winter vegetables are emerging.

Figure 8. Cabbage looper larva.
Cabbage looper moths lay single, dome-shaped eggs on the under side of older leaves. A single female may lay 275 to 350 eggs. Eggs will darken as they age, and will hatch in 2 to 5 days. The larvae are light green in color and have a distinctive white stripe along each side of the body. The larvae have two sets of legs in the front of the body and three sets of fatter, unjointed prolegs at the rear. They move in a "looping" manner, arching the middle portion of the body as they move forward. Two to four weeks are required for full development to a 5th instar larva. Cabbage looper pupae appear as greenish to brown pupas wrapped in a delicate white cocoon of fine threads usually attached to the underside of the leaf. Pupation usually takes 10 to 16 days. The moth is mottled brown in color, and has a small silvery spot (sometimes a figure 8) near the middle of its front wing. Cabbage loopers may have 3 to 5 generations per year.
Damage: Loopers damage plants by eating ragged holes in leaves, and sometimes working their way into heads. They also cause damage by contaminating marketable portions with their bodies and frass. High populations can chew seedlings severely enough to kill them or slow growth enough to inhibit uniform maturing of the crop, but most economic damage occurs after heading. Young plants between thinning and heading can tolerate substantial feeding by loopers and other caterpillars without loss of yield or quality. Heads contaminated with loopers, or tunneled into by loopers are not marketable.
Management and Control: Monitoring for cabbage looper and other Lepidopterous pests should involve sampling plants twice a week once seedling emergence begins. When populations appear to be increasing, check more often. Follow the guidelines used for monitoring beet armyworms. On lettuce, monitor for eggs and larvae of loopers while checking for other caterpillar pests that feed on leaves and heads. Action thresholds are similar to those of beet armyworm: treat seedlings or small plants when populations of small loopers are large enough to stunt growth. If other Lepidopterous species are present, also include them in this total. Between thinning and heading, treat if the worm population reaches one larva per 50 plants. During head formation, treat if sample counts exceed one larva per 25 plants. Cabbage loopers are especially sensitive to B.t.s. Including a B.t. with insecticide applications targeting beet armyworms will usually control any cabbage loopers present.
Heliothinae
Corn earworm, Helicoverpa zea (Boddie) and Tobacco budworm, Heliothis virescens (Fabricius)
Description and Life History: Heliothinae are very destructive pests of many crops including corn, cotton, tomatoes, lettuce, soybeans, and grain sorghum. Heliothinae frequently move into lettuce from surrounding crops, particularly corn and cotton. This pest occurs statewide but is most common in central and western Arizona. Although, the tobacco budworm is the predominant species in lettuce in the low desert areas, both corn earworm and tobacco budworm are very similar in appearance and biology, and their management strategies in lettuce are the same.
Corn earworm moths vary in color but most have light grayish-brown front wings with irregular lines and dark areas toward the tip of the wings. The hindwings are white with irregular dark spots. The front wings of the tobacco budworm moth are pale olive in color with three narrow, dark, oblique bands. The hindwing is white with a reddish-brown border. The wingspan of both moths is approximately 1 1/2 inches. Female moths lay their eggs singly on lettuce leaves. Eggs are white when laid but develop a dark red or brown ring around the top within 24 hours. They darken before hatching as the larvae develop inside. Deeper ridges and a more hemispherical shape distinguish Heliothinae eggs from those of cabbage or alfalfa loopers. One female moth will lay 500 to 3,000 eggs. Heliothinae prefer to lay their eggs toward the crown portion of the plant, in the terminal growth. Eggs hatch in 2 to 10 days.
Along the backs of newly hatched Heliothinae are discrete rows of tubercles or small bumps, with one or two hairs protruding from each. Heliothinae larvae are cannibalistic and will eat siblings and other Lepidopterous larvae. Thus, they are usually not found in close proximity to other worms. They prefer to feed on the terminal growth or heads of lettuce plants. Heliothinae larvae usually develop distinct stripes as they mature, but overall color of caterpillars is variable. The tubercles and hairs remain obvious on older larvae that are dark colored but are less visible on lighter ones. In addition to the larger hairs and tubercles, Heliothinae have tiny short spines covering large portions of the skin that can be seen with a 10X hand lens. These tiny spines distinguish the corn earworm and the closely related tobacco budworm from all caterpillars likely to be found on lettuce. Heliothinae larvae will feed 2 to 4 weeks and molt five times before pupating. They will pupate in the soil for 10 to 25 days.
Damage: When early-season populations are high, Heliothinae can decimate seedling stands of lettuce. Damage to seedlings is similar to that caused by beet armyworms. Heliothinae are much more likely to bore into lettuce heads than other Lepidoptera larvae, rendering the heads unmarketable. Larvae feed in the plant's crown leaving holes and gouges in the midrib, sometimes killing the growing point. Potential for damage decreases as the seedlings grow; economic damage is not common between thinning and head formation.
Once heads form, large Heliothinae larvae will usually bore into the head. Larvae may enter the head from any point, although they usually burrow in from the top half. When burrows begin under or between the wrapper leaves, the infestation may not be noticed until the head is harvested. Once inside the head, Heliothinae are protected and difficult to control with insecticides.
Management and Control: Delaying lettuce planting until after nearby cotton is defoliated may help in reducing Heliothinae pressure. Follow the guidelines for monitoring other Lepidopterous larvae. Lettuce seedlings are very susceptible to Heliothinae damage. As soon as seedlings emerge, check for Heliothinae eggs. Be particularly attentive if the lettuce field borders cotton. Dark-colored eggs will be those near hatching. It is best to time insecticide applications at, or just after peak egg hatch. Lettuce should be treated if a significant number of Lepidoptera eggs and larvae are present.
Between thinning and heading, lettuce plants can tolerate up to one Lepidopterous larva per 50 plants. Be sure to correctly identify the species in your field. Biology and management guidelines for the tobacco budworm are essential the same as for corn earworm, except that the range of available insecticides is more limited for the budworm. Repeated insecticide treatments are often required to maintain low population levels.
During head formation, check for Heliothinae larvae every time you visit the field by pulling back the wrapper leaves or even cross sectioning some heads. Once heads form, keep Heliothinae populations as low as possible. One larva boring into a head will cause that head to be unmarketable. Treat when Lepidopterous larvae average one per 25 plants. Time applications to control the caterpillars during hatching and before they enter the protected areas within the head.
Green Peach Aphid, Myzus persicae (Sulzer)
Description and Life History: The green peach aphid is considered the most economically important aphid pest on lettuce in the southwestern United States (Fig. 9). The green peach aphid is generally considered to be a pest in the spring.

Figure 9. Green peach aphid adult.
Winged green peach aphid adults have a black/brown head and thorax. Their abdomen is light green or red with a black/brown mottling. At the base of each antenna of many aphids is a small bump called a tubercle. In green peach aphids these tubercles are pronounced and converging inwardly, while similar species tubercles are less pronounced or diverging. Wingless adults are light green or red with the same antennal bumps. Nymphs appear as smaller versions of the wingless adults.
The life cycle of the green peach aphid is typical for aphids. In southern climates it reproduces asexually. Populations consist entirely of female aphids giving live birth to female progeny. This type of reproduction gives aphids a tremendous reproductive capacity. One female can easily give birth to 80 to 100 young in her lifetime of about 30 days. In colder climates green peach aphids overwinter as eggs laid on peach trees (or occasionally on apricot or prune).
In response to crowding by other aphids or declining host plant quality, migratory (winged) forms are produced that move to new hosts (weeds or crops). This ordinarily occurs in the early spring.
Damage: Green peach aphid damages lettuce primarily by acting as a contaminant. Packers will accept very little aphid contamination. Additionally, green peach aphids serve as vectors for alfalfa mosaic virus, lettuce mosaic virus, and beet western yellows virus that affects lettuce. The viruses that attack lettuce have not been a problem in recent years. Predicting virus problems is virtually impossible as it is dependent upon when aphid flights take place and levels of inoculum. Little if anything can be done to prevent the disease even if we could make accurate predictions.
Management and Control: Lettuce planted so that harvest will occur during February and March, should be prophylactically treated with a soil-applied systemic insecticide at planting. Other plantings can rely on foliar materials for control. Check fields twice weekly, but most intensely beginning mid-January. Green peach aphid prefers the underside of the older leaves. After thinning and before heading, colonies of ten or fewer aphids, can be tolerated. Once lettuce nears head formation, green peach aphids cannot be tolerated. The key to controlling green peach aphid is to prevent the formation of large colonies. Adequate control is often difficult to achieve with foliar sprays, and follow-up scouting should be performed to determine if another insecticide application is necessary. Green peach aphids are often most numerous in fields containing mustards weeds, cheeseweed and members of the goosefoot family. Control of these weeds may help prevent the buildup of green peach aphid.
Thrips
Western Flower Thrips, Frankliniella occidentalis (Pergande) and Onion Thrips, Thrips tabaci Lindeman
Description and Life History: Thrips are present season long in most lettuce fields, they are usually most abundant during the spring after temperatures have warmed. Thrips can build up in weedy areas, onions, wheat or non-irrigated pastures, moving to lettuce in large numbers when other hosts begin to dry down. Most thrips are slender, light-colored yellowish-brown insects not more than 1/16 inch long as an adult. They are very active and will rapidly scurry around on the plant tissue. The adults usually have distinctive red eyes and feathery wings. The small white bean shaped eggs are laid in the plant tissue. The eggs will hatch in 2 to 7 days. Nymphs resemble adults except for their smaller size, lack of wings, and are paler in color. Nymphs will pass through four instars in 15 to 30 days. The two later instars do not feed and are passed in the soil or litter as pupae beneath the plants.
Because of their difference in ease of control, it is important to be able to distinguish between western flower and onion thrips. The western flower thrips is very difficult to define, because it has many color forms. The females range in color from light yellow, yellow with brown blotches on the body, to dark brown, while the male western flower thrips are light yellow (Fig. 10). Adult western flower thrips are about 1/20 inch in length. The immature stages are generally light yellow in color. Western flower thrips have reddish orange ocellar pigmentation and eight segmented antennae. Additionally, western flower thrips have long black setae at the front and back of the pronotum (Fig. 11).

Figure 10. Adult western flower thrips.
In the United States, the onion thrips reproduce asexually through parthenogenisis. There are no male onion thrips in the U.S. The female onion thrips is slightly smaller than female western flower thrips, being only 1/25 inch long. Its body is yellow with brown blotches on the thorax and abdominal terga. The legs of the onion thrips are yellowish brown, while the antennae appear striped. Antennal segment I and the base of segments III to V are brownish~white. The rest of the antenna is brown. The onion thrips has seven-segmented antennae and their ocellar pigment is generally gray. Immature onion thrips are generally light yellow in color. Adult light-colored forms are predominant during warm temperatures while brown forms occur when it is cool. The onion thrips has long back setae at the back of the pronotum, but lack them at the front (Fig. 11).
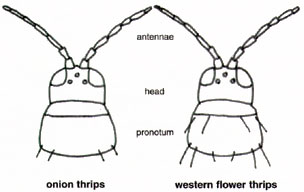
Figure 11. Onion thrips have seven segmented antannae and
long black setae only at the back of the pronotum, while the western flower
thrips have eight segmented antennae and long black setae at the front
and back of the pronotum.
Damage: Thrips feeding causes the leaves of slow growing seedlings to become wrinkled and distorted. However, this injury may be of little importance if temperatures are warm enough for the plants to outgrow it. Feeding also causes brown scarring of leaves, giving them a speckled or scorched appearance. This type of damage is often confused with wind burn or blown sand damage, but can be distinguished by numerous black flecks scattered over the discolored area -these are the feces of thrips. On young plants, severely damaged leaves dry out and drop. Damage is often not noticed until the thrips population has declined or disappeared. Romaine lettuce is especially susceptible to thrips damage and should be closely monitored for thrips. Near harvest, thrips can act as contaminates to harvestable portions.
Management and Control: Thrips outbreaks are often associated with fields heavily infested with weedy mustard weeds or fields near commercial plantings of mustard, alfalfa or wheat. Many thrips populations, particularly western flower thrips, are resistant to some insecticides and adequate control is often difficult to achieve. Chemical control should only be used when thrips populations are extremely high or product contamination is of concern. Pyrethroids used alone should be avoided for control of western flower thrips, because they are suspected of causing populations to flare, but have been shown to be effective against onion thrips.
Diseases
Significant fungal diseases of lettuce in Arizona include bottom rot caused by Rhizoctonia solani, leaf drop caused by Sclerotinia minor and S. sclerotiorum, downy mildew caused by Bremia lactucae and powdery mildew caused by Erysiphe cichoracearum. Another major disease of lettuce in Arizona is big vein, which is caused by double-stranded ribonucleic acid that is presumed to be a virus-like agent. Fungicides can be effective management tools for lettuce diseases caused by fungi. Refer to specific fungicide product labels for comprehensive information concerning use of each product.
Other diseases of minor importance that occasionally occur
in Arizona will not be discussed here. For a more comprehensive treatment
of all diseases occurring on lettuce, refer to the University of Arizona
Cooperative Extension Publication number 191050, entitled "Diseases
of Lettuce in Arizona."
Bottom rot
This disease is caused by Rhizoctonia solani. This fungus is one of the most common soilborne plant pathogens in agricultural soils of Arizona and survives by colonizing soil organic matter. Bottom rot is most widespread on early season lettuce that matures from mid- to late-autumn with disease developing on plants that have headed and are nearly mature. In Arizona, bottom rot can be found when environmental conditions favor disease development and bottom leaves of lettuce plants touch the soil.
Symptoms: There are no obvious above ground symptoms of bottom rot under the arid environmental conditions prevalent in Arizona. Mature lettuce plants appear normal until they are cut at harvest. Infected plants display sunken, reddish-brown lesions of varying depths and sizes on leaf petioles and midribs that touch the soil (Fig. 12). White to brownish mycelium grows over these lesions. The fungus can move upward from leaf to leaf until the entire head is colonized. Affected leaves are removed in the field prior to packing. Plants with extensive deep lesions are usually left in the field. Leaf tissue infected with Rhizoctonia solani may also be invaded by soft rot bacteria, resulting in a slimy decay that may cause additional losses of infected lettuce heads.

Figure 12. Head lettuce with bottom rot infection.
Disease Cycle: Rhizoctonia solani can survive indefinitely in soil because it is an active colonizer of soil organic matter. Rhizoctonia is spread by any means that moves soil from one place to another. The pathogen can be carried long distances on infected plant parts, which can bear sclerotia of the fungus. The sclerotia can germinate in damp soil and enter plants through wounds or through stomata on leaves touching the soil. The optimum temperature for growth of the pathogen is, with minimum and maximum temperatures for mycelial growth of 41 and 96° F.
Management and control: Growers should avoid planting
lettuce immediately after other crops known to be susceptible to Rhizoctonia,
such as alfalfa. Fungicides, such as iprodione (Rovral) and vinclozolin
(Ronilan), can be effective management tools for bottom rot on lettuce.
Fungicide application should be initiated early, just after thinning,
to achieve maximum suppression of pathogen activity.
For
more information on bottom rot from the UA Plant Pathology web site.
Leaf drop
Leaf drop of lettuce in Arizona is caused by two pathogenic fungi, Sclerotinia minorand S. sclerotiorum. Leaf drop was first described in Arizona in 1925 and probably occurs in all lettuce- production areas of the world when cool moist conditions exist. The fungi can persist in soil for long periods of time in the form of overseasoning sclerotia. S. sclerotiorum and to a lesser extent S. minor can cause disease in a wide variety of different plants, including many vegetable crop plants.
Symptoms: The name 'leaf drop' best describes the prominent and most obvious symptom of this disease (Fig. 13). The pathogen usually invades the main stem or upper root, causing a soft, dark, watery decay. The destruction of stem tissue causes a rapid wilt, collapse and death of infected plants. In Arizona, this symptom is likely to be observed as the plants gain enough size to cover the majority of the plant bed. Dense masses of white fungal growth appear on the surface of rotted tissue near the soil surface. Hard black irregularly shaped fungal structures called sclerotia are produced on and in the rotted host tissue. The pathogen can be identified by the size of sclerotia produced on decayed lettuce tissue; S. minor produces small sclerotia 1/16 to 1/8 inch in diameter, whereas the larger sclerotia produced by S. sclerotiorum are usually from 1/4 to 3/8 inch in diameter and sometimes larger.

Figure 13. Lettuce affected by leaf drop.
Disease Cycle: These pathogens carry-over from season to season as active mycelium in dead plant tissue and as sclerotia in soil. Mycelium emerging from germinating sclerotia of S. minorand S. sclerotiorum can infect lettuce plants directly through senescent lower leaves and through root tissue near the soil surface. Sclerotia of S. sclerotiorum can also germinate in shaded areas on the soil surface during wet weather by producing small flesh-colored flat or cup-shaped structures called apothecia, which are borne on short stalks approximately 1/4 inch in length. Apothecia forcibly release ascospores into the air for a period of 2 to 3 weeks. These ascospores are carried by air currents and deposited on healthy lettuce plants, which subsequently become infected. Sclerotia germinate in moist soil during cool weather. Sclerotinia can cause infection from 32 to 82° F, with an optimum temperature range between 60 and 70° F. Sclerotia may survive up to 10 years in dry soil, whereas they decay in moist soil and may rot in less than a year.
Management and Control: There are several management
strategies that can be implemented to minimize losses due to leaf drop
caused by both species of Sclerotinia. 1) Avoid excessive irrigations
which tend to wet the top of lettuce beds; this is especially important
when lettuce plants begin to cover a majority of the bed surface. Remember
that wet soil stimulates sclerotial germination and plant infection. 2)
Deep plowing tends to bury sclerotia, which promotes rotting of these
fungal propagules and reduces their ability to germinate and cause infection.
3) Crop rotations with resistant crops such as corn and grasses should
be used in problem fields. 4) Chemical management tools, such as iprodione
(Rovral) and vinclozolin (Rovral), can provide effective disease control
when applied promptly after thinning according to label recommendations.
For
more information on leaf drop from the UA Plant Pathology web site.
Downy mildew
Downy mildew, caused by the obligate parasitic fungus Bremia lactucae, usually can be found in lettuce fields each year in Arizona. However, both the incidence and severity of the disease are governed by the frequency and duration of cool moist conditions required for disease development. Free moisture on the leaf surface is essential for spore germination and infection, but not growth of the fungus within the leaf. A limiting factor for development of downy mildew in desert production areas is the rare occurrence of persistent cool weather combined with high humidity and rainfall.
Symptoms: The first symptoms of downy mildew on lettuce are pale yellow regions on the upper side of older wrapper leaves with a corresponding white fluffy growth, which contains the spores of the pathogen, on the lower leaf surface (Fig. 14). The infected areas are limited by leaf veins. The spores (sporangia) of the fungus are produced singly on branched treelike structures. Affected tissue will eventually turn brown in color.

Figure 14. The fungal mycelium of downy mildew is evident
only on the underside of the leaf.
Disease Cycle: Sporangia from leaf spots are released into the air and blown long distances by prevailing winds to healthy leaf tissue. In the presence of free water on the leaf surface, the spore germinates and can penetrate and infect epidermal cells within three hours.
Following establishment of the fungus in leaf tissue, fruiting stalks emerge through stomates on the affected lower leaf surface, branch repeatedly and produce an abundant crop of sporangia, which are released into the air to cause new infections. In Arizona this cycle often is suppressed by dry weather that follows rainy periods. Minimum, optimum, and maximum temperatures for infection are approximately 40, 50 to 72, and 86° F, respectively. The pathogen can overseason in crop debris and soil as thick-walled oospores.
Management and Control: Downy mildew can be successfully
managed by planting varieties of lettuce with tolerance or resistance
to the pathogen when available. Several different pathovars of Bremia
exist, some of which can infect varieties that were formerly resistant.
Timely application of fungicides when environmental conditions are favorable
can effectively suppress disease development. Some fungicides that are
effective for control of downy mildew on lettuce include maneb, fosetyl-Al
(Aliette), and metalaxyl (Ridomil). Several experimental fungicides have
shown promise in controlling downy mildew and may be available in the
future. To achieve maximum benefits from fungicides, they must be applied
when environmental conditions favor disease development and before the
appearance of disease symptoms. Insensitivity to the systemic fungicide
metalaxyl has been documented for some time. Alternating use of different
chemistries as well as utilization of other resistance management strategies
are strongly encouraged to impede the development of resistance to other
fungicides by Bremia lactucae.
For
more information on downy mildew from the UA Plant Pathology web site.
Powdery mildew
Powdery mildew, caused by the obligate parasitic fungus, Erysiphe cichoracearum, may occur on lettuce from January through April in desert production areas. This disease primarily affects plants approaching maturity, occurs in the absence of free water on leaf surfaces and is often confused with downy mildew.
Symptoms: Symptoms begin as small tufts of fungal growth on upper or lower leaf surfaces. As the disease progresses, the leaf area covered by the fungus increases, eventually coating the entire leaf with a powdery or dusty appearing growth (Fig. 15). Masses of spores produced in chains are found with the fungal growth on leaf surfaces.

Figure 15. Powdery mildew infects both upper and lower
leaf surfaces.
Powdery and downy mildew can be differentiated by the following characteristics: 1) the powdery mildew pathogen produces spores in long chains on a single stalk on both sides of a lettuce leaf, whereas the downy mildew pathogen produces spores singly on branched stalks only on the underside of infected leaves; 2) powdery mildew can spread over the entire leaf surface and appears as a white dusty growth, whereas downy mildew lesions are delineated by leaf veins; 3) powdery mildew can occur in the absence of free water on the leaf surface, whereas downy mildew only occurs during wet humid weather when free water is present on leaf surfaces.mildew.
Disease Cycle: Conidia (spores) from infected plant tissue can be blown long distances to infect healthy leaves. Temperatures between 65-77° F are most favorable for germination of conida and subsequent infection of leaves. Relative humidity of 85% or above is required for infection, growth of mycelium and sporulation. From 4-10 days are required from initial infection to production of a new crop of conidia. Low light intensity tends to favor development of powdery mildew.
Management and Control: If available, planting of
resistant varieties is advised for lettuce that will mature in late winter
to early spring in desert production areas. Application of sulfur to leaf
surfaces before the onset of disease when environmental conditions are
favorable can effectively inhibit disease development. New chemistries
have been tested and found to effectively control the development of powdery
mildew on lettuce. Some of these materials could be available in the future
as chemical disease management tools.
For
more information on powdery mildew from the UA Plant Pathology web site.
Viral Diseases
There are six viral diseases that have the potential of causing moderate to serious crop losses on southwestern Arizona lettuce plantings. They are; Lettuce Infectious Yellows Virus (LIYV), Lettuce Mosaic Virus (LMV), Beet Western Yellows Virus (BWYV), Cucumber Mosaic Virus (CMV), Alfalfa Mosaic Virus (AMV) and Big Vein. Of these diseases, big vein is most common. For any of these viruses, there are no known chemical controls. Controlling insect vectors, eliminating other sources of host plant material, maintaining sound cultural and crop rotation practices and using only indexed seed are the only viable controls available to date for most of these viral diseases.
Big vein
Big vein is caused by a virus-like agent that is carried or vectored by the soil-borne, root inhabiting fungus Olpidium brassicae. The disease was first reported in the Imperial Valley of California in 1934.

Figure 16. Lettuce infected with the Big Vein virus.
Symptoms: Big vein was named for its most prominent symptom, a pronounced clearing of the chlorophyll adjacent to major veins giving the appearance of enlarged veins (Fig. 16). Leaf veins are prominent when held up to bright light. Leaves of infected plants are more upright and have a more ruffled puckered appearance compared to healthy plants. In the field, conspicuous symptoms normally occur approximately one month after seeding and only when air temperatures during the day are below 65° F. Constant temperatures above approximately 70° F prevent big vein symptom development. Infected plants mature more slowly, may fail to form a head or produce small inferior heads.
Disease Cycle: Olpidium brassicae serves two important functions in the big vein disease. Zoospores of this fungus are produced under saturated soil conditions and transport the pathogen internally and inoculate it into lettuce root cells. Resting spores of Olpidium carry the pathogen internally and allow it to survive in soil from crop to crop for a minimum of eight years. Olpidium has a wide host range, including wild species of lettuce, celery, radish, onion and broccoli. In Aguila, Arizona the incidence of big vein was over 60% in a field first planted to lettuce, providing compelling evidence that the vector and the virus-like agent were present in the soil prior to planting. Disease incidence is higher in heavy textured poorly drained soils where zoospore production is favored by saturated soil conditions.
Management and Control: An effective management strategy
for big vein is to plant varieties of lettuce that are tolerant to the
disease. Fields with a history of big vein should not be planted with
a lettuce crop that will mature when temperatures favor infection and
symptom expression. No cost-effective chemical soil treatments are available.
For
more information on big vein and other viral-type diseases from the UA
Plant Pathology web site.
Harvest and Post-Harvest
Depending on variety, planting slot, temperature, salts and other abiotic and biotic factors, lettuce will generally be ready for harvest in approximately 65 for fall-planted crops and 100 days winter-planted crops. Lettuce is timed for harvest when quality is at its peak. However when demand for lettuce exceeds supply, the harvest timing may be slightly shifted toward an early or later harvest by as much as 7 days.
Because of the extensive labor involved, harvesting is the greatest expense in lettuce production. Lettuce in Arizona is harvested using three handling techniques: naked packed, film wrapped, or bulked. Field packaged lettuce may be packed "naked" in the carton, meaning they have no plastic wrapper; film-wrapped in perforated or non-perforated cellophane; or bagged in perforated plastic bags. Perforated cellophane is used to prevent moisture accumulation and extend shelf life. Bagged lettuce is packaged in perforated plastic bags, with usually six heads per bag.
Most of the lettuce grown in Arizona is field packed, meaning that the product is harvested, packaged in the field, and shipped to market with no further processing. Because most lettuce undergoes little processing, great emphasis is placed on producing a high quality product. It is essential that the product be free of pest damage and contamination at harvest.
Regardless of the field packaging technique used, harvesting methods are essentially the same (Fig. 17). A harvesting crew consists of eight groups of three individuals, working behind a harvest aid or wrapping machine. Each group consists of two people cutting heads, and one person packaging.

Figure 17. Harvest crew cutting and packaging lettuce near
Yuma, AZ.
Harvesters cut the lettuce near the soil surface with a long knife then trim unwanted leaves usually leaving 4 to 5 wrapper leaves. The packer will package the lettuce in a cardboard carton. Each carton will contain two layers of lettuce heads. Each layer is usually composed of 12 heads. The bottom layer is package butt-down or cut side down, while the upper layer is packaged butt-up to prevent latex sap from dripping onto the foliage. A typical carton of lettuce will weigh about 50 lbs. The packer is also responsible for wrapping the lettuce with cellophane or bagging when required. After harvest, the lettuce is transported to a cooling shed and distribution center where it is stored at 35 to 36° F. Although lettuce storage life under these conditions is 16 to 20 days, almost all lettuce is shipped with 48 hours.
Bulk harvesting of lettuce has become more popular in recent years, because of the popularity of prepackage salads. Specific fields may be grown for bulk harvesting, or in many cases, bulk harvesters will follow the wrapping machines, harvesting heads not suited for field packaging. Bulk harvesters consist of large crews of people who cut heads and place them in large cardboard or plastic bins. Bins of high quality bulked lettuce may be slated for the fast food restaurant industry. However, most are transported to salad plants where they are sorted, washed with a dilute chlorine solution or fumigated with ozone, and then chopped for prepackaged or ready-made salads in sealed plastic bags. This type of processing is known as "value-added" packaging. Similar to non-processed lettuce, value-added packaged lettuces are stored prior to transport at 35 to 36° F. Value-added lettuce has a shelf life of 12 to 14 days, and are usually shipped from the salad plant within 1 to 2 days. All value-added packaging has an expiration date printed on it.
Yields of lettuce grown in Arizona vary widely from 650 to 1000 cartons per acre, depending on demand, supply, and quality. When demand is high and supply short, harvesters will often cut heads of lesser size or quality that would normally be bulked or left in the field.
Lettuce market prices vary wildly depending on demand and availability because it has a short shelf live and is sold at harvest. Growers do not have the luxury of waiting for a favorable price. The lettuce market ultimately determines how much insect damage and contamination a packer will accept. When the lettuce price is low and there is an abundance of lettuce being harvested, packers are very discriminating and only high quality lettuce with no insect damage or contamination is accepted. However, when the price is high and lettuce availability is low, packers will often accept lettuce that is of poorer quality, or has some cosmetic maladies. Because the price of lettuce at harvest is unpredictable, growers manage their crops as if only high quality lettuce will be accepted.
For Yuma lettuce growers, lettuce prices are traditionally best from early-November to early-December, and towards the end of March and in April when lettuce harvesting activity in the Salinas, Huron and Bakersfield areas of California is low. Arizona growers will commonly alter planting and harvesting schedules to exploit times when weather or other factors have hampered lettuce production in California. Unlike fiber and grain crops, the lettuce market is much more volatile and is not as globally driven. Localized inclement weather, diseases or insect outbreaks can also greatly influence the domestic price of lettuce. In Arizona, the average monthly lettuce price in dollars per carton from 1990-96 averaged $12.78 in November, $15.26 in December, $11.14 in January, $10.35 in February, $14.68 in March, and $16.80 in April. Seasonal averages were $9.25 in 1990-91, $9.80 in 1991-92, $14.40 in 1992-93, $9.16 in 1993-94, $20.70 in 1994-95 and $13.10 in 1995-1996.
Because lettuce is a perishable commodity, growers cannot store their product when prices are undesirable. Thus, lettuce growers are at the immediate mercy of the current price in the lettuce market. Lettuce production is a high risk, high capital endeavor that requires keen planning, marketing, and production practices to be successful.
Issued in furtherance of Cooperative Extension work, acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director Cooperative Extension, College of Agriculture and Life Sciences, The University of Arizona.
The University of Arizona is an equal opportunity, affirmative action institution. The University does not discriminate on the basis of race, color, religion, sex, national origin, age, disability, veteran status, or sexual orientation in its programs and activities.
Any products, services, or organizations that are
mentioned, shown, or indirectly implied in this web document do not imply
endorsement by The University of Arizona.
Information provided by:
David L. Kerns, dkerns@ag.arizona.edu, IPM Specialist
Michael E. Matheron, matheron@ag.arizona.edu, Plant Pathologist
John C. Palumbo, jpalumbo@ag.arizona.edu, Entomologist
Charles A. Sanchez, sanchez@ag.arizona.edu, Soil Scientist
David W. Still, Seed Physiologist
Barry R. Tickes, btickes@ag.arizona.edu, Extension Agent, Yuma County
Kai Umeda, kumeda@ag.arizona.edu, Extension Agent, Maricopa County
Mark A. Wilcox, Extension Agent, Yuma County
University of Arizona, Tucson, Arizona.
Material written February 1999.
Crop Mgmt | Soil Mgmt | Irrigation | Varieties | Quality
Home | Vegetables
document located at: http://cals.arizona.edu/crops/vegetables/cropmgt/az1099.html
Copyright © 2001 University of Arizona,
College of Agriculture and Life Sciences
webmaster: jsjones@ag.arizona.edu