|
Arabadopsis studies: a faster |
|||||
|
Finding traits for salt, drought and cold tolerance By Susan McGinley
|
|||||
|
|
Many of the world’s food crops grow in marginal soil. As desertification
and erosion increase, cropland is reduced, forcing people to grow crop
plants under conditions they are not traditionally adapted to, including
high salt concentrations in soil and water, low water availability,
and cold temperatures. As crops fail and human populations begin to starve, the search for
better adapted plants becomes more urgent. A novel technique for gene
identification, under development at the University of Arizona, may
help speed the development of more stress-hardy crop plants. Jian-Kang
Zhu, a professor in the Department of Plant Sciences, has spent the
last five years identifying and isolating genes from arabidopsis plants—a
type of crucifer, the plant group that includes broccoli, cauliflower
and cabbage—that can be used for biotechnological applications
to make hardier crops. His research concentrates on how this particular
plant adapts to stressful environments. “We wanted to identify, out of 20,000 to 30,000 genes in a typical
plant, how many actually help the plant cope with stressful environments,”
Zhu says. “Using arabidopsis, we have identified about a dozen
genes so far that are of critical importance—several for salt,
quite a few for chilling and freezing, and a few for drought.”
For some of these genes, the university is applying for patents. Zhu needed to find a way to communicate with plants, to know what was
going on inside them. He knew that genes in a plant could be turned
off and on, expressing themselves in reaction to the environment, but
wasn’t sure exactly when this happened. “By the time you see
drought symptoms in the plant, it’s usually too late to do anything
about it,” he says. He has turned to fundamental science, specifically
to genome studies (the analysis of the full gene complement in a set
of chromosomes) to do this. Zhu and his team use certain gene promoters in arabadopsis plants to
help turn the genes on and off in response to stress. He puts the promoter
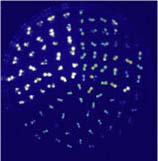
together with something to make it glow—in this case, the firefly
enzyme luciferase—and ntroduces it into the arabadopsis plant to
make it glow steadily when subjected to stress. This way he can identify
the genes that control stress responses and isolate them. He puts the
small plants, which are anchored on growth media plates, under temperature,
water, or salt stress. In the case of chilling or freezing stress, the
plants go into a refrigerator, to trigger the cold-response gene. Since the luciferase enzyme induces a glow too weak to be seen by the
naked eye, Zhu uses a camera to view the steady glow. To test which
genes are responsible for the glow, he puts chimeric agrobacterium genes
into the plant to induce mutations in the genes and make them nonfunctional,
then observes the consequences of that plant’s response to the
environment. He keeps screening to find plants that are reacting abnormally,
and separates them from normal plants. “With 30,000 genes, you want to know which is important,”
Zhu says. “If a plant is not glowing, or glowing too brightly,
we know a particular gene is involved.” This process is painstaking,
and yields results slowly, but works much faster than traditional breeding
methods, which can take years as different plant generations are grown
out and evaluated. “The important thing is to know which genes are important for each trait,” Zhu says. They may express themselves in both the leaves and the roots. “When we find those genes, we put them back into normal arabadopsis plants to make them better. If they do, they are ready to be put into crops, but doing that takes more effort.” While arabadopsis takes six to eight weeks to reach maturity, crop plants take much longer. For this reason, Zhu is sending those genes to cooperative institutions where researchers are testing them on crop plants. “We’ve provided several genes to Cornell University to put into rice,” he says. “We’ve also worked together with a group at Purdue to introduce some of these genes into tomatoes.” In addition, commercial firms are interested in licensing patents to use these genes. Zhu cautions that it could still take years to make sure the genes express themselves consistently.
|
||||